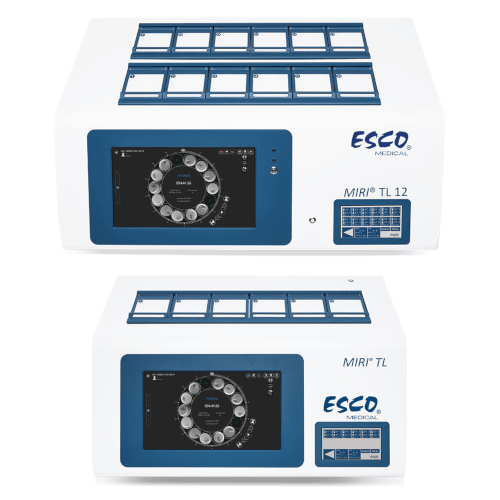
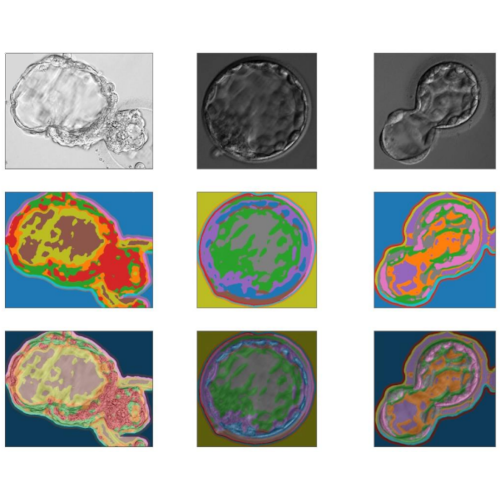
Available with 6 or 12 chambers, the MIRI® Time-Lapse Incubator is a multiroom incubator that comes equipped with a microscope and camera. The integrated camera and microscope offer real-time, high-quality time-lapse images of embryo development, eliminating the need for manual microscopy and the potential risks associated with removing embryos from the safe environment of the incubation chamber. With time-lapse embryo monitoring, detailed morphokinetic data throughout the entire developmental process can be gathered, providing insights not available through traditional spot microscopic evaluation. This allows for the observation of all critical events, aiding in the identification of healthy embryos with the highest potential for implantation, and ultimately resulting in higher pregnancy rates.
The process of embryo annotation has been simplified with an innovative annotation system, which revolves around “events” located on the left side of the interface. The pre-programmed events list includes t2, t3, t4, t5, t6, t7, t8, morula, blastocyst, and early blastocyst, but the software allows for complete customization of each event. Users can modify the pre-programmed events list by adding other criteria using the advanced parameters in the unit’s Settings menu. To make the process even more user-friendly, an Ideal Time function has been added, represented by a colored circular band on the edge of annotated events. This feature indicates the ideal timings for each event, making it easier to compare the actual timing of embryo development with the ideal timing.
In addition to the integrated timelapse and annotation functionality, these incubators feature all of the advantages standard to the MIRI product line, including distinct chambers which ensure the maintenance of environmental conditions, a heated lid to enhance temperature regulation and recovery, an onboard gas mixer, and an optional integrated SafeSENS pH system.
UNIT
- Available in 6 or 12 Chambers
- 6 individually-controlled chambers
- Integrated gas mixer; CO2 and O2 Control
- Extremely reliable and easy-to-use
- Best-priced timelapse incubator on the market
- Optional integrated SafeSENS pH System
- Available with Fairtility CHLOE AI System